In a formal letter to Centers for Medicare & Medicaid Services
(CMS), the American College of Chest Physicians (CHEST) endorsed the
need to make a key change to the designation of delirium that recognizes
it as a major complication or comorbidity. CHEST is grateful to the
American Delirium Society for leading this charge.
The proposed change would more accurately represent the clinical
importance of delirium and the tremendous costs associated with it. The
suggested change will make the complexity designation consistent with
toxic (G92) and metabolic (G93.41) encephalopathy (TME) which is implied
in cases of delirium as an underlying factor.
CHEST, which represents a large portion of the community of critical
care clinicians, recognizes that this change is essential to our ability
to improve the clinical care and outcomes of patients who are
cognitively vulnerable.
The full letter can be found below.
Dear Center for Medicare & Medicaid Services,
We write to request that causally specified delirium be designated as major
complication or comorbidity (MCC), which would make its complexity
designation consistent with toxic (G92) and metabolic (G93.41)
encephalopathy (TME).
This change is essential to recognizing the clinical importance of delirium
and, crucially, the tremendous costs associated with it.1,2
Placing delirium and encephalopathy on par with TME in terms of
reimbursement is intended to facilitate systematic efforts to detect
delirium as recommended across specialties and settings,3-6
thereby enhancing awareness of delirium and its dire impact on patients,
their families, care delivery, and healthcare systems.7
The ultimate goal of this change is to improve the clinical care and
outcomes of cognitively vulnerable patients.
Executive summary
We request that all causally specified delirium diagnoses be designated as
MCC, consistent with TME. This is justified because a delirium diagnosis in
the DSM-5-TR8 and ICD-109 requires both a defined
clinical syndrome plus attribution to a direct physiological cause. In other
words, a diagnosis of causally specified delirium implies an underlying TME.
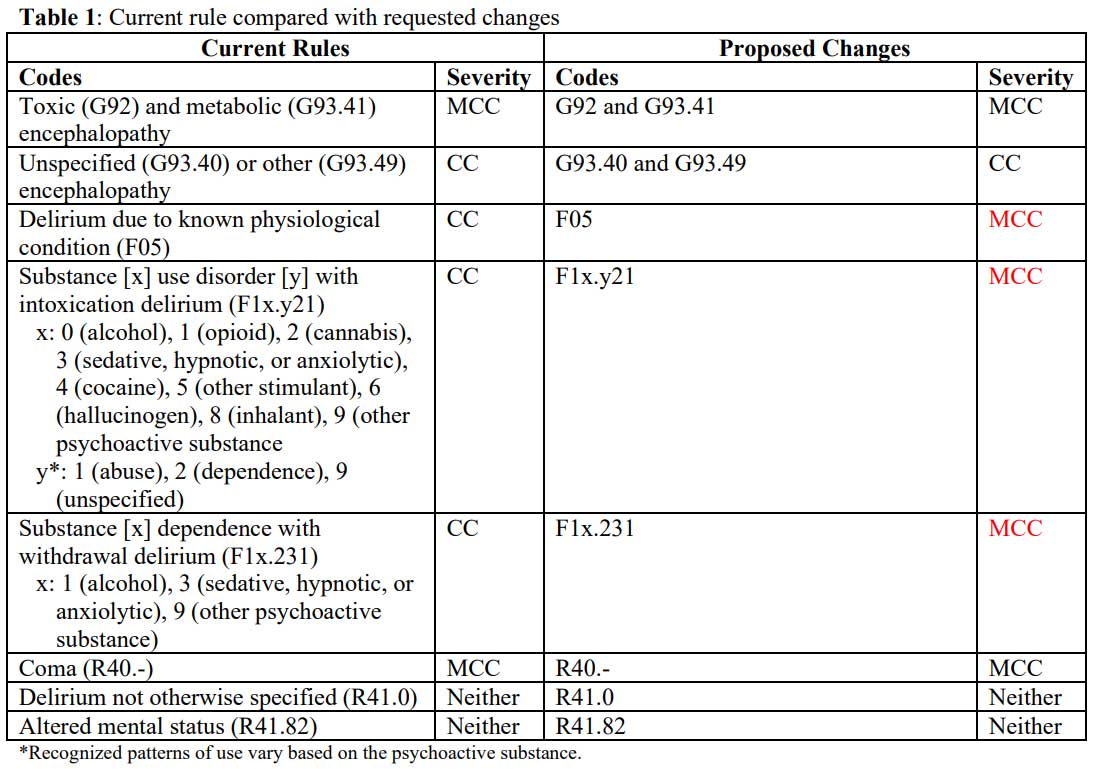
Our requests are tabulated below (Table 1), with requested
changes are in red.
Background
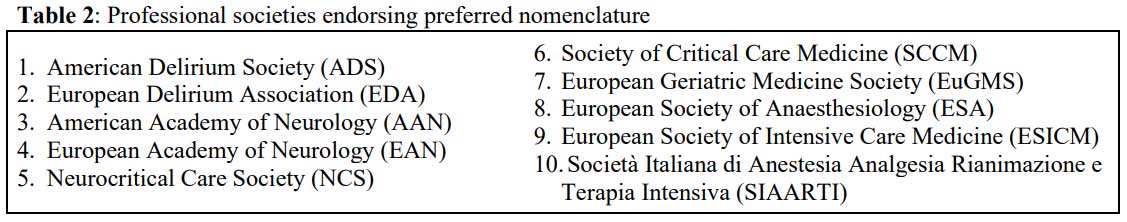
Endorsed by 10 medical societies (Table 2), a 2020 position
statement on preferred nomenclature of delirium and acute encephalopathy,
clarified definitions of “acute encephalopathy” and “delirium,” as well the
relationship between them.1 The statement provides the following
definitions:
Acute encephalopathy: “a rapidly developing (in less than 4
weeks) pathobiological brain process which is expressed clinically as either
subsyndromal delirium, delirium or coma.”
The diagnostic codes for acute encephalopathy include toxic, metabolic,
other, and unspecified encephalopathy.
Subsyndromal delirium: “acute cognitive changes that are
compatible with delirium, but do not fulfil all DSM-5 delirium
criteria”
Delirium: “a clinical state defined according to the
criteria of the DSM-5” (n.b., the current edition is
DSM-5-TR8)
Coma: “a state of severely depressed responsiveness defined
using diagnostic systems such as the Glasgow Coma Score (GCS) or the Full
Outline of UnResponsiveness (FOUR) score”
The clinical syndromes of subsyndromal delirium, delirium, and coma alert
the clinician to an underlying acute encephalopathy, as they are the
cognitive evidence of a pathobiological brain process disrupting global
consciousness. Conversely, acute encephalopathy would not be suspected, let
alone diagnosed, in the absence of such clinical syndromes. That is,
the presence of a delirium-spectrum syndrome entails the presence of an
underlying acute encephalopathy,10 as all editions of the DSM have included a
diagnostic criterion for delirium, variably worded, requiring that the
cognitive disturbances be attributable to the “direct physiological
consequence of another medical condition, substance intoxication or
withdrawal, or exposure to a toxin, or is due to multiple etiologies.”
Delirium in relation to acute encephalopathy
Reliability is necessary for high-quality care; however, the diagnosis of
acute encephalopathy risks being unreliable on its own because it lacks
operationalized diagnostic criteria. Further, to our knowledge, no clinical
severity thresholds have ever been validated to define acute encephalopathy
caseness. Several EEG patterns may support a diagnosis of acute
encephalopathy,11,12 but without reliable criteria this
diagnosis will be diagnosed based on nonspecific changes in mental status.
At the same time, these same EEG findings for acute encephalopathy are
correlated with delirium severity,13 which is important because
delirium is a well validated clinical construct with reliable
operationalized criteria.14 The severity of the
delirium-spectrum illness from subsyndromal delirium to delirium to coma is
the bedside clinical analog of an EEG that indexes the severity of the
underlying acute encephalopathy. Clinical complexity increases and outcomes
worsen incrementally with the severity of mental status change: subsyndromal
delirium with moderate impact,15,16 delirium with major impact,7,17,18 and coma with severe impact.19
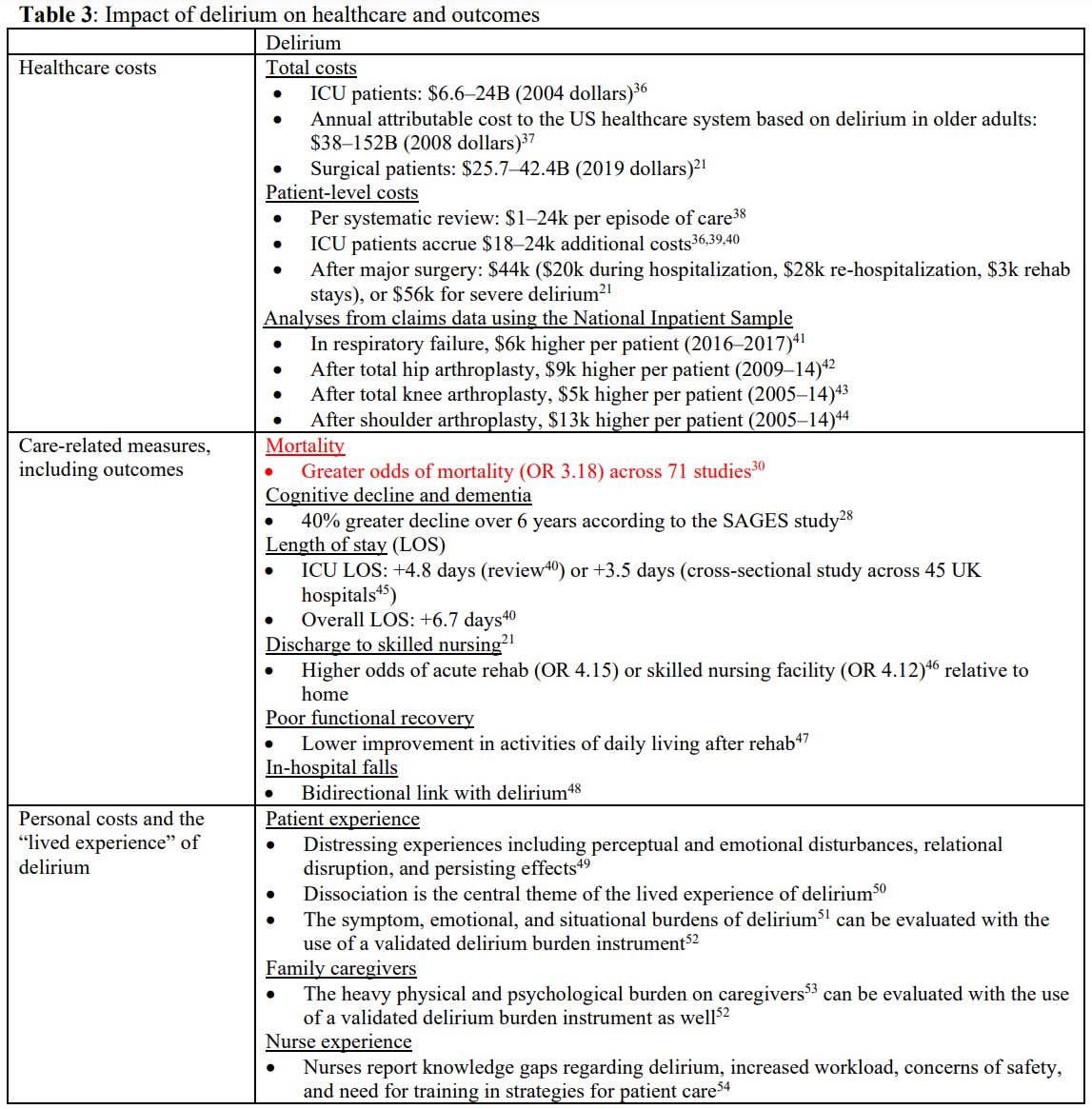
A robust literature details the impact of delirium on care complexity and
costs,20,21 readmissions,22 rates of functional
decline,23,24 institutionalization,25 cognitive
decline,26-28 dementia,29 and mortality,30,31
yet curiously there is no parallel in the “toxic/metabolic encephalopathy”
literature (Table 3). Delirium continues to attract
increasing32 and increasingly serious international attention33,34 for its tremendous public health impact. Further, the
relationship between delirium and Alzheimer’s disease and related dementias
is prioritized in research.35
The models of delirium and acute encephalopathy each have a rich tradition,2 yet a variety of historical, institutional, and even
clinician-level factors have conspired against their integration.10
This is despite the fact that they represent interdependent aspects of a
shared set of acute neurocognitive syndromes. Among the most pressing
reasons for their division, though, is economic.55 Currently,
at each level of specification, each acute encephalopathy code
(G-series) is designated as a higher complexity of illness than the
corresponding delirium (F-series) code even though causally specified
delirium codes provide even greater specificity (Table 4).
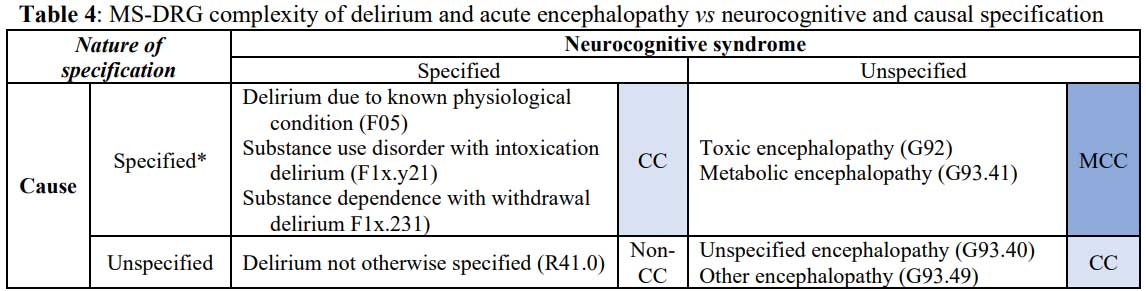
* ICD-10-CM requires that one “Code first the underlying
physiological condition” for F05, whereas the relevant substance is denoted
by the ones digit in the diagnostic code for intoxication denoted x
in the table and withdrawal deliria. The tenths digit denoted by y
in the table refers to the substance use pattern. ICD-10-CM
requires that one “Code first, if applicable, drug induced (T36-T50) or use
(T51-T65) to identify toxic agent” for G92 but does not have similar coding
requirements for G93.41.56
The coding landscape in the U.S. bears this out: in 2011 encephalopathy
diagnoses outnumbered delirium nearly 4:1 but in 2018 the ratio was more
than 13:1,57 which one suspects is due in large part due to the
higher reimbursement.55 [Note, in a supplementary
document, we provide an updated analysis based on the National Inpatient
Sample for 2019—the last full year before the COVID pandemic—that considers
the impact of our proposal.] The effects of the current system, by
prioritizing reimbursement of G-series codes over F-series codes, may
inadvertently reward institutions that are savvier with such billing
incentives, either institutions whose practitioners have been taught to code
the corresponding G-series code preferentially or those with separate coding
departments. The issue of upcoding delirium to TME was among the key
allegations in a whistleblower lawsuit brought by Integra Med Analytics LLC
against Providence Health and Services.58 Although the case was
ultimately dismissed,59 it clearly highlights this disparity. In
the initial filing, the Integra complaint noted that “[e]ncephalopathy is a
term for brain disease or damage to the brain where the brain is regarded as
‘altered in its structure or function.’ The telltale symptom is an altered
mental state, but altered mental state alone is insufficient for diagnosing
encephalopathy” (as quoted in the minutes from the case60).
However, one considers it telling that no clinical definition for
encephalopathy appears to be offered by the plaintiff in this case.
On the origins of the disparity
Why, when the MS-DRG was being developed, might acute encephalopathy—and, in
particular, TME—have performed like an MCC whereas delirium performed like a
CC?55 Whereas these diagnoses had been on par in terms of
reimbursement prior to the MS-DRG system, they were clearly not being used
interchangeably in hospitals. How might coding practices at the time have
introduced an artifact of severity?
The first observation applies to both delirium and TME diagnoses. That is,
only a fraction of patients with TME/delirium ever receive either diagnosis.
A review of coding in 2018 revealed that the combined prevalence of both was
roughly 3%,57 yet prospective studies find substantially higher
values. For instance, it is rare to find studies that report a lower than
10% prevalence of delirium after major surgery, and more than half of
patients in critical care develop delirium.61 Delirium rates
across acute medical settings vary by population and, typically, age,
pre-existing cognitive impairment, and overall morbidity.62
Therefore, in view of this likely type II error in national coding, the
question becomes, “What clinical factors would lead to an artifact of
differential complexity?”
Consider TME. First, neurologists have historically favored the term TME
whereas most other specialties have favored delirium2 (notably,
coding in internal medicine has increasingly favored TME for reasons that
are addressed in this proposal1). Returning to the mid-2000’s
when the MS-DRG system was developed, we should consider the role of
neurology in clinical care. For a neurologist to be involved in a patient’s
care either as the primary or consulting service, the mental status change
is typically to such a degree that it warrants independent clinical
attention for neurological evaluation, often being accompanied by focal
neurological findings. Diagnostic consideration would naturally include
encephalitis, seizure disorders, space-occupying lesions, cerebrovascular
accidents, and the like. Such an evaluation often involves head imaging, EEG
evaluation, or even lumbar puncture. As such, TME diagnoses at the time were
all but certainly enriched with more severe clinical scenarios.
Next, consider delirium. The vast majority of delirium is never diagnosed,
which means that it would be more informative to ask, “When is delirium
diagnosed?” rather than “When is it undiagnosed?”63-66 In
general, a diagnosis of delirium tends to be made when there is
hyperactivity and, in particular, behavioral disturbances. The far more
common hypoactive presentations go either undiagnosed
or preferentially diagnosed as TME
. Additionally, the tradition of the neurologists Victor and Adams was to
reserve the diagnosis of delirium for hyperactive states and encephalopathy
for hypoactive ones.2 However, of the motoric subtypes of
delirium, hypoactive delirium is consistently associated with worse clinical
outcomes, including greater risk of mortality.67-71 That is, the
data used to create the MS-DRG likely would have included a small group of
largely hyperactive delirium as the complication or comorbidity in question,
leaving the majority of patients with the more severe hypoactive delirium in
the non-delirium reference group, thereby creating a false impression that
delirium is of lower complexity.
Delirium and the nine guiding principles for reconsideration of its
MS-DRG complexity designation
Delirium is a textbook example that maps onto the nine guiding principles to
evaluate when considering a potential change to CMS coding and
reimbursement.72 However, an epistemic principle, even more
foundational than the nine, is added as a preceding item as number zero
below because the ability to detect a condition reliably is necessary for
consistent detection and clinical intervention.
0. Delirium can be diagnosed reliably whereas TME cannot be diagnosed
reliably without a defined threshold.
-
An independent TME diagnosis currently lacks demonstrated
reliability whereas delirium has reliable, operationalized
diagnostic criteria.8
-
Delirium instruments are available to detect delirium reliably
across settings. In particular, the suite of Confusion Assessment
Method delirium assessment instruments has been validated
extensively both categorically and as severity instruments,73-78 leading the CAM to be the most widely used
set of instruments to detect delirium worldwide. Further, the
Ultra-Brief CAM79 can equip a large range of
healthcare clinicians to detect delirium reliably in less than a minute
and a half, with most patients screening negative in less than a minute
on an initial 2-item screener.80
-
Recognizing the link between delirium and acute encephalopathy
encourages diagnostic reliability by standardizing clinical
definitions and allowing for systematic detection efforts.81
1. “Involves a chronic illness with susceptibility to exacerbations or
abrupt decline.”
-
Acute and chronic forms of cognitive impairment share a
bidirectional relationship such that preexisting cognitive
impairment increases the risk of delirium and delirium increases the
risk of subsequent cognitive decline and dementia.82
The relationship between delirium and Alzheimer’s disease and
related dementias remains of critical importance to older adults,
especially within the Age-Friendly Health Systems and Geriatric
Surgery Verification Program initiatives.
-
Delirium superimposed on dementia may be a particularly virulent
condition and appears to involve the acceleration of decline and
increased risk of mortality.83
-
Further, these proposed changes, by requiring clinical specificity,
bear similarity to the recent changes in dementia diagnostic codes
that provide greater specification of neuropsychiatric disturbances
beyond simply “with/without behavioral disturbances.”84
2. “Serves as a marker for advanced disease states across multiple
different comorbid conditions.”
-
Delirium is common across hospital settings and comorbidities, in
particular occurring in roughly a third of hospitalized older
adults.81
-
Prioritizing delirium detection facilitates the recognition of
mental status changes heralding clinical deterioration for prompt
recognition and redress of contributing clinical factors.
-
Regarding its broad applicability to clinical care, mental status
changes may be regarded as a vital sign.85,86
3. “Reflects systemic impact.”
-
Delirium is an essential element of the Age-Friendly Health System,
with deep interconnections with each of the 4M’s,87 and Geriatric Verification Program88
initiatives.
-
Delirium in post-acute care settings is associated with more than
twice the risk of 30-day mortality, 40% increased risk of 30-day
hospital readmission, and 40% lower rate of discharge home within 30
days.89
-
Table 3
(see above) details many aspects of delirium’s systemic impact.
4. “Post-operative/post-procedure condition/complication impacting
recovery.”
-
“Postoperative delirium” is the uniformly recommended term to
describe acute neurocognitive disturbances after surgery.90
-
Apart from the nearly universal experience of postoperative pain,
delirium is arguably the most common complication after major
surgery and has an outsized impact on postoperative recovery.91 As described in the clinically focused review
in NEJM by Dr. Marcantonio,91
delirium is associated with: a 2- to 5-fold increased risk of
postoperative complications, including risk of death, an additional
2–5 days length of stay, a 3-fold increased risk of institutional
placement at discharge, poor functional recovery, and new dementia
diagnosis.
-
The healthcare costs and sequelae attributable to delirium consider
not only incremental costs during the index episode of care but also
care utilization over the following year.21
5. “Typically requires higher level of care (that is, intensive
monitoring, greater number of caregivers, additional testing, intensive care
unit care, extended length of stay).”
-
We refer, again, to Table 3 above.
6. “Impedes patient cooperation or management of care or both.”
-
Patient experience must be considered when discussing the
distinctions between delirium and TME. TME draws attention to
underlying pathobiology, but it does not specify the clinical
manifestation of that disturbance and the diverse ways that its
neuropsychiatric disturbances routinely impede care delivery and
recovery.10 A diagnosis of delirium, on the other hand,
centralizes the patient’s experience, drawing attention to the
importance of the patient’s mental status and care engagement.50,92,93
-
A diagnosis of delirium requires a clinician to characterize a
patient’s mental status.
This information is essential so that clinicians understand a
patient’s ability to engage meaningfully in care decisions, have
discussions about their care with clinicians and loved ones, and
participate productively in various aspects of care.
-
Additionally, identifying delirium as “delirium” encourages
evaluation and monitoring for neuropsychiatric disturbances that
increase the risk of danger, including impulsivity, risk of falls,
inadvertent self-extubation or line removals, and other elements of
compromised care.94
7. “Recent (last 10 years) change in best practice, or in practice
guidelines and review of the extent to which these changes have led to
concomitant changes in expected resource use.”
-
To our knowledge, there is no defined treatment pathway for the TME
diagnoses; however, several guidelines published in the past 10
years exist for delirium, both in the United States (e.g., by the
American Geriatrics Society,6 the Society for Critical Care Medicine,4 Cochrane
Database of Systematic Reviews,95 and
American Psychiatric Association [update currently in process]) and
internationally (e.g., the National Institute for Health and Clinical
Excellence,96 the Scottish Intercollegiate
Guidelines Network,97 Australian Delirium
Care Standard,98 European Society of
Anaesthesiology,99 Association of Scientific
Medical Societies of Germany,100 and
Japanese Psycho-Oncology Society and Japanese Association of Supportive
Care in Cancer101).
8. “Denotes organ system instability or failure.”
-
Although the term “acute brain failure” is discouraged by the recent
multi-society statement on nomenclature (for its redundancy rather
than for its inaccurate connotations),1 this term was the subtitle of Lipowski’s first
of two delirium monographs.102 Nomenclature
aside, there is no question that delirium represents a global
disturbance in cognition as a form of “failure of neurocognition”103
and global dysfunction.
-
Delirium is associated with markers of brain damage including on
postmortem neuropathology,104 elevated neurofilament light chain (marker of
axonal damage),105,106 elevated serum tau,107 and
several inflammatory markers known to index neural injury.108
9. “Represents end of life/near death or has reached an advanced stage
associated with systemic physiologic decompensation and debility.”
-
Delirium is a state of systemic physiological decompensation
associated with advanced illness. Delirium at the end of life
(“terminal delirium”) is a common expression of advanced disease
that can cause patients and their families distress, and dangerous
behavioral disturbances.109
Rectifying reimbursement actively facilitates prevention efforts
Finally, we consider that these changes would facilitate delirium
prevention
efforts as well. Delirium is costly, and its complexity designation should
be commensurate with its economic impact. However, delirium prevention
efforts work, preventing roughly 40% of delirium.110,111 The
costs associated with delirium not only justify its designation as MCC but
also encourage widespread delirium prevention efforts. Care bundles such as
the AGS CoCare® HELP 112,113 and the Society of Critical Care
Medicine’s ICU Liberation A-to-F bundle114,115 have been shown
to be both effective at preventing delirium and cost effective. How
might a change in delirium’s complexity designation incentivize adoption of
such delirium care bundles?
Despite the effectiveness of delirium prevention bundles, encouraging
delirium prevention efforts alone, without changing the complexity
designation of delirium, would not be enough. This is because most delirium
is not preventable; this includes both delirium prevalent on admission
(inherently not preventable116) and the 60% of incident delirium
that is not currently preventable with modern delirium bundles.111
Designating delirium as MCC would signal to hospitals the costs associated
with delirium, thereby leading to a greater awareness of its scope and
healthcare impact. It would also be apparent that additional reimbursement
for delirium as MCC is modest relative to the cost savings realized by
delirium prevention.113 This is because the incremental increase
in reimbursement for delirium as MCC is relevant
only for the portion of patients without a separate qualifying MCC
. Insurance companies would also have reason to incentivize hospitals to
implement delirium preventive care. Further still, parallel efforts by the
Age-Friendly Health Systems initiative117 along with the
increasingly integrated AGS CoCare® HELP,118 the Society of
Critical Care Medicine’s ICU Liberation A-F Bundle,119 and the
American College of Surgery’s Geriatric Surgery Verification Program,120
and the integrative work of the American Delirium Society121
provide a collective counterbalance in leading and advocating for delirium
prevention.
In fact, one imagines a potentially stronger argument for how addressing the
current reimbursement disparity between delirium and acute encephalopathy
could advance not only best practices but also facilitate delirium
prevention and improve patient care. We are currently witnessing an epidemic
of burnout among clinicians in acute medical settings,122 fueled
in part by the behavioral complexity of hospitalized patients with cognitive
disorders. Currently, however, the burden of delirium bundles and other
non-pharmacological interventions to prevent delirium is being placed on
nurses, practitioners, and other clinicians providing direct patient care.
Based on the change in reimbursement outlined in our request, improved
revenue for delirium is expected to more than offset the costs of investing
in dedicated staff to ensure the consistent, successful implementation of
delirium bundles, thereby offloading already-overstretched clinicians.
Again, the cost equation of these bundles strongly suggests that, given
sufficient ‘energy of activation’ to see them implemented, they would be
financially self-sustaining.113
Clinical impact statement
Our proposal represents the logical conclusion of understanding the
integrated nature of delirium and acute encephalopathy. We expect that this
proposal, which aligns reimbursement with a robust scientific literature and
clinical practice guidelines, will facilitate improved patient care and
outcomes by way of encouraging enhanced delirium detection and actionable
delirium clinical pathways.
Many clinicians and healthcare systems are simply unaware of the scope and
impact of delirium. Those who know the scope and have attempted to implement
delirium detection efforts and delirium pathways know how hard it is to get
buy-in for these efforts, or to develop sustainable quality improvement
projects that do not depend on a single person or a small group of dedicated
champions to keep it alive. Our proposal, if accepted, would provide
healthcare institutions with appropriate and justified incentives to provide
appropriate support to patients with the greatest cognitive and functional
vulnerability—specifically, those at risk for complications and poor
outcomes. In view of such a change, one envisions education efforts to
clinical staff about what delirium is, what it looks like, and why it
matters, as well as practical tools including optimized electronic health
records for delirium functionality and the availability of
non-pharmacological interventions.
Summary
We are requesting that causally specified delirium codes be designated as
MCC. This proposal is aligned with the principles of value-based care and
the aim of the MS-DRG system to accurately account for the variance in
healthcare costs.
Respectfully submitted,
Mark Oldham, MD
President-Elect, American Delirium Society
The following organizations are in support of this proposal
(in alphabetical order) :
-
Academy of Consultation-Liaison Psychiatry
-
American Association for Geriatric Psychiatry
-
American Delirium Society
-
American Geriatrics Society
-
American Thoracic Society
-
Association of Medicine and Psychiatry
-
Society of Critical Care Medicine
-
Society of Hospital Medicine (preliminarily)
Additionally, the American Academy of Neurology and American Psychiatric
Association
Additional Supplements
-
“An Analysis of Annual Nationwide Coding of CC Specified Delirium and
MCC Encephalopathy.” This analysis estimates the number of hospital
encounters this proposal would affect annually.
-
Slooter AJC, Otte WM, Devlin JW, et al. Updated nomenclature of delirium
and acute encephalopathy: statement of ten Societies. Intensive care
medicine 2020;46(5):1020-1022.
-
Oldham MA, Holloway RG. Delirium disorder: Integrating delirium and
acute encephalopathy. Neurology 2020;95(4):173-178.
References
1. Slooter AJC, Otte WM, Devlin JW, et al. Updated nomenclature of
delirium and acute encephalopathy: statement of ten Societies. Intensive
Care Med 2020;46(5):1020-1022. DOI: 10.1007/s00134-019-05907-4.
2. Oldham MA, Holloway RG. Delirium disorder: Integrating delirium
and acute encephalopathy. Neurology 2020;95(4):173-178. DOI:
10.1212/WNL.0000000000009949.
3. National Institute for Health and Clinical Excellence. Delirium:
prevention, diagnosis and management in hospital and long-term care.
(Clinical guideline) (https://www.nice.org/uk/guidance/cg103).
4. Devlin JW, Skrobik Y, Gelinas C, et al. Clinical Practice
Guidelines for the Prevention and Management of Pain, Agitation/Sedation,
Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU.
Crit Care Med 2018;46(9):e825-e873. DOI: 10.1097/CCM.0000000000003299.
5. Scottish Intercollegiate Guidelines Network (SIGN). Risk
reduction and mangaement of delirium.
6. American Geriatrics Society Expert Panel on Postoperative
Delirium in Older A. American Geriatrics Society abstracted clinical
practice guideline for postoperative delirium in older adults. J Am Geriatr
Soc 2015;63(1):142-50. DOI: 10.1111/jgs.13281.
7. Wilson JE, Mart MF, Cunningham C, et al. Delirium. Nat Rev Dis
Primers 2020;6(1):90. DOI: 10.1038/s41572-020-00223-4.
8. American Psychiatric Association. Diagnostic and statistical
manual of mental disorders: DSM-5-TR. Arlington, VA: American Psychiatric
Association Publishing, 2022.
9. World Health Organization (WHO). The ICD-10 Classification of
Mental and Behavioural Disorders: World Health Organization, 1993.
10. Oldham MA. Delirium disorder: Unity in diversity. Gen Hosp
Psychiatry 2022;74:32-38. DOI: 10.1016/j.genhosppsych.2021.11.007.
11. Sutter R, Kaplan PW. Clinical and electroencephalographic
correlates of acute encephalopathy. J Clin Neurophysiol 2013;30(5):443-53.
DOI: 10.1097/WNP.0b013e3182a73bc2.
12. Sutter R, Kaplan PW, Valenca M, De Marchis GM. EEG for Diagnosis
and Prognosis of Acute Nonhypoxic Encephalopathy: History and Current
Evidence. J Clin Neurophysiol 2015;32(6):456-64. DOI:
10.1097/WNP.0000000000000164.
13. Tesh RA, Sun H, Jing J, et al. VE-CAM-S: Visual EEG-Based Grading
of Delirium Severity and Associations With Clinical Outcomes. Crit Care
Explor 2022;4(1):e0611. DOI: 10.1097/CCE.0000000000000611.
14. Kimchi EY, Neelagiri A, Whitt W, et al. Clinical EEG slowing
correlates with delirium severity and predicts poor clinical outcomes.
Neurology 2019;93(13):e1260-e1271. DOI: 10.1212/WNL.0000000000008164.
15. Cole MG, Ciampi A, Belzile E, Zhong L. Persistent delirium in
older hospital patients: a systematic review of frequency and prognosis. Age
Ageing 2009;38(1):19-26. DOI: 10.1093/ageing/afn253.
16. Serafim RB, Soares M, Bozza FA, et al. Outcomes of subsyndromal
delirium in ICU: a systematic review and meta-analysis. Crit Care
2017;21(1):179. DOI: 10.1186/s13054-017-1765-3.
17. Rosgen BK, Krewulak KD, Stelfox HT, Ely EW, Davidson JE, Fiest KM.
The association of delirium severity with patient and health system outcomes
in hospitalised patients: a systematic review. Age Ageing
2020;49(4):549-557. DOI: 10.1093/ageing/afaa053.
18. Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH.
Days of delirium are associated with 1-year mortality in an older intensive
care unit population. Am J Respir Crit Care Med 2009;180(11):1092-7. DOI:
10.1164/rccm.200904-0537OC.
19. Singh B, Murad MH, Prokop LJ, et al. Meta-analysis of Glasgow coma
scale and simplified motor score in predicting traumatic brain injury
outcomes. Brain Inj 2013;27(3):293-300. DOI: 10.3109/02699052.2012.743182.
20. Leslie DL, Inouye SK. The importance of delirium: economic and
societal costs. J Am Geriatr Soc 2011;59 Suppl 2:S241-3. DOI:
10.1111/j.1532-5415.2011.03671.x.
21. Gou RY, Hshieh TT, Marcantonio ER, et al. One-Year Medicare Costs
Associated With Delirium in Older Patients Undergoing Major Elective
Surgery. JAMA Surg 2021;156(5):430-442. DOI: 10.1001/jamasurg.2020.7260.
22. LaHue SC, Douglas VC, Kuo T, et al. Association between Inpatient
Delirium and Hospital Readmission in Patients >/= 65 Years of Age: A
Retrospective Cohort Study. J Hosp Med 2019;14(4):201-206. DOI:
10.12788/jhm.3130.
23. Rudolph JL, Inouye SK, Jones RN, et al. Delirium: an independent
predictor of functional decline after cardiac surgery. J Am Geriatr Soc
2010;58(4):643-9. DOI: 10.1111/j.1532-5415.2010.02762.x.
24. Oldenbeuving AW, de Kort PL, Jansen BP, Algra A, Kappelle LJ, Roks
G. Delirium in the acute phase after stroke: incidence, risk factors, and
outcome. Neurology 2011;76(11):993-9. DOI: 10.1212/WNL.0b013e318210411f.
25. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom
P, van Gool WA. Delirium in elderly patients and the risk of postdischarge
mortality, institutionalization, and dementia: a meta-analysis. JAMA
2010;304(4):443-51. DOI: 10.1001/jama.2010.1013.
26. Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive
decline in Alzheimer disease. Neurology 2009;72(18):1570-5. DOI:
10.1212/WNL.0b013e3181a4129a.
27. Pandharipande PP, Girard TD, Jackson JC, et al. Long-term
cognitive impairment after critical illness. N Engl J Med
2013;369(14):1306-16. DOI: 10.1056/NEJMoa1301372.
28. Kunicki ZJ, Ngo LH, Marcantonio ER, et al. Six-Year Cognitive
Trajectory in Older Adults Following Major Surgery and Delirium. JAMA Intern
Med 2023;183(5):442-450. DOI: 10.1001/jamainternmed.2023.0144.
29. Pereira JV, Aung Thein MZ, Nitchingham A, Caplan GA. Delirium in
older adults is associated with development of new dementia: a systematic
review and meta-analysis. Int J Geriatr Psychiatry 2021. DOI:
10.1002/gps.5508.
30. Aung Thein MZ, Pereira JV, Nitchingham A, Caplan GA. A call to
action for delirium research: Meta-analysis and regression of delirium
associated mortality. BMC Geriatr 2020;20(1):325. DOI:
10.1186/s12877-020-01723-4.
31. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of
mortality in mechanically ventilated patients in the intensive care unit.
JAMA 2004;291(14):1753-62. DOI: 10.1001/jama.291.14.1753.
32. McCoy TH, Jr. Mapping the Delirium Literature Through
Probabilistic Topic Modeling and Network Analysis: A Computational Scoping
Review. Psychosomatics 2019;60(2):105-120. DOI: 10.1016/j.psym.2018.12.003.
33. Khachaturian AS, Hayden KM, Devlin JW, et al. International drive
to illuminate delirium: A developing public health blueprint for action.
Alzheimers Dement 2020;16(5):711-725. DOI: 10.1002/alz.12075.
34. Oh ES, Akeju O, Avidan MS, et al. A roadmap to advance delirium
research: Recommendations from the NIDUS Scientific Think Tank. Alzheimers
Dement 2020;16(5):726-733. DOI: 10.1002/alz.12076.
35. Wang S, Lindroth H, Chan C, et al. A Systematic Review of Delirium
Biomarkers and Their Alignment with the NIA-AA Research Framework. J Am
Geriatr Soc 2020. DOI: 10.1111/jgs.16836.
36. Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with
delirium in mechanically ventilated patients. Crit Care Med
2004;32(4):955-62. DOI: 10.1097/01.ccm.0000119429.16055.92.
37. Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK.
One-year health care costs associated with delirium in the elderly
population. Arch Intern Med 2008;168(1):27-32. DOI:
10.1001/archinternmed.2007.4.
38. Kinchin I, Mitchell E, Agar M, Trepel D. The economic cost of
delirium: A systematic review and quality assessment. Alzheimers Dement
2021;17(6):1026-1041. DOI: 10.1002/alz.12262.
39. Vasilevskis EE, Chandrasekhar R, Holtze CH, et al. The Cost of ICU
Delirium and Coma in the Intensive Care Unit Patient. Med Care
2018;56(10):890-897. DOI: 10.1097/MLR.0000000000000975.
40. Dziegielewski C, Skead C, Canturk T, et al. Delirium and
Associated Length of Stay and Costs in Critically Ill Patients. Crit Care
Res Pract 2021;2021:6612187. DOI: 10.1155/2021/6612187.
41. Taha A, Xu H, Ahmed R, et al. Medical and economic burden of
delirium on hospitalization outcomes of acute respiratory failure: A
retrospective national cohort. Medicine (Baltimore) 2023;102(2):e32652. DOI:
10.1097/MD.0000000000032652.
42. Yang Q, Wang J, Huang X, Xu Y, Zhang Y. Incidence and risk factors
associated with postoperative delirium following primary elective total hip
arthroplasty: a retrospective nationwide inpatient sample database study.
BMC Psychiatry 2020;20(1):343. DOI: 10.1186/s12888-020-02742-6.
43. Yang Q, Wang J, Chen Y, Lian Q, Shi Z, Zhang Y. Incidence and risk
factors of postoperative delirium following total knee arthroplasty: A
retrospective Nationwide Inpatient Sample database study. Knee
2022;35:61-70. DOI: 10.1016/j.knee.2022.02.006.
44. Yang Q, Fu J, Pan X, et al. A retrospective analysis of the
incidence of postoperative delirium and the importance of database selection
for its definition. BMC Psychiatry 2023;23(1):88. DOI:
10.1186/s12888-023-04576-4.
45. Geriatric Medicine Research C. Delirium is prevalent in older
hospital inpatients and associated with adverse outcomes: results of a
prospective multi-centre study on World Delirium Awareness Day. BMC Med
2019;17(1):229. DOI: 10.1186/s12916-019-1458-7.
46. Zipser CM, Spiller TR, Hildenbrand FF, et al. Discharge
Destinations of Delirious Patients: Findings From a Prospective Cohort Study
of 27,026 Patients From a Large Health Care System. J Am Med Dir Assoc
2022;23(8):1322-1327 e2. DOI: 10.1016/j.jamda.2022.01.051.
47. Madrigal C, Kim J, Jiang L, et al. Delirium and Functional
Recovery in Patients Discharged to Skilled Nursing Facilities After
Hospitalization for Heart Failure. JAMA Netw Open 2021;4(3):e2037968. DOI:
10.1001/jamanetworkopen.2020.37968.
48. Sillner AY, Holle CL, Rudolph JL. The Overlap Between Falls and
Delirium in Hospitalized Older Adults: A Systematic Review. Clin Geriatr Med
2019;35(2):221-236. DOI: 10.1016/j.cger.2019.01.004.
49. Kuusisto-Gussmann E, Hockelmann C, von der Luhe V, Schmadig R,
Baltes M, Stephan A. Patients' experiences of delirium: A systematic review
and meta-summary of qualitative research. J Adv Nurs 2021;77(9):3692-3706.
DOI: 10.1111/jan.14865.
50. Gaete Ortega D, Papathanassoglou E, Norris CM. The lived
experience of delirium in intensive care unit patients: A meta-ethnography.
Aust Crit Care 2020;33(2):193-202. DOI: 10.1016/j.aucc.2019.01.003.
51. Schmitt EM, Gallagher J, Albuquerque A, et al. Perspectives on the
Delirium Experience and Its Burden: Common Themes Among Older Patients,
Their Family Caregivers, and Nurses. Gerontologist 2019;59(2):327-337. DOI:
10.1093/geront/gnx153.
52. Racine AM, D'Aquila M, Schmitt EM, et al. Delirium Burden in
Patients and Family Caregivers: Development and Testing of New Instruments.
Gerontologist 2019;59(5):e393-e402. DOI: 10.1093/geront/gny041.
53. Assa AH, Wicks MN, Umberger RA. Family Caregivers' Experience of
Patients With Delirium in Critical Care Units: A State-of-the-Science
Integrative Review. Am J Crit Care 2021;30(6):471-478. DOI:
10.4037/ajcc2021394.
54. Thomas N, Coleman M, Terry D. Nurses' Experience of Caring for
Patients with Delirium: Systematic Review and Qualitative Evidence
Synthesis. Nurs Rep 2021;11(1):164-174. DOI: 10.3390/nursrep11010016.
55. Oldham MA. Follow the Money: The Widening Coding Disparity Between
Acute Encephalopathy and Delirium. J Acad Consult Liaison Psychiatry 2022.
DOI: 10.1016/j.jaclp.2022.02.004.
56. American Medical Association. ICD-10-CM 2022 the Complete Official
Codebook with Guidelines: American Medical Association, 2021.
57. Franks JA, Anderson JL, Bowman E, Li CY, Kennedy RE, Yun H.
Inpatient Diagnosis of Delirium and Encephalopathy: Coding Trends 2011-2018.
J Acad Consult Liaison Psychiatry 2022. DOI: 10.1016/j.jaclp.2021.12.006.
58. Integra Med Analytics v. Providence Health Services. U. S.
District Court Central District of California - Western Division; 2018.
59. Pierson B. Integra loses bid to revive its data-based FCA case v.
Providence. Reuters(R).
(https://www.reuters.com/business/legal/integra-loses-bid-revive-its-data-based-fca-case-v-providence-2021-05-13/).
60. Integra Med Analytics v. Providence Health and Services. U. S.
District Court Central District of California; 2019.
61. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly
people. Lancet 2014;383(9920):911-22. DOI: 10.1016/S0140-6736(13)60688-1.
62. Ormseth CH, LaHue SC, Oldham MA, Josephson SA, Whitaker E, Douglas
VC. Predisposing and Precipitating Factors Associated With Delirium: A
Systematic Review. JAMA Netw Open 2023;6(1):e2249950. DOI:
10.1001/jamanetworkopen.2022.49950.
63. Inouye SK. The dilemma of delirium: clinical and research
controversies regarding diagnosis and evaluation of delirium in hospitalized
elderly medical patients. Am J Med 1994;97(3):278-88. DOI:
10.1016/0002-9343(94)90011-6.
64. van Eijk MM, van Marum RJ, Klijn IA, de Wit N, Kesecioglu J,
Slooter AJ. Comparison of delirium assessment tools in a mixed intensive
care unit. Crit Care Med 2009;37(6):1881-5. DOI:
10.1097/CCM.0b013e3181a00118.
65. Elie M, Rousseau F, Cole M, Primeau F, McCusker J, Bellavance F.
Prevalence and detection of delirium in elderly emergency department
patients. CMAJ 2000;163(8):977-81.
(https://www.ncbi.nlm.nih.gov/pubmed/11068569).
66. Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM, Jr. Nurses'
recognition of delirium and its symptoms: comparison of nurse and researcher
ratings. Arch Intern Med 2001;161(20):2467-73.
(http://www.ncbi.nlm.nih.gov/pubmed/11700159).
67. de Rooij SE, Schuurmans MJ, van der Mast RC, Levi M. Clinical
subtypes of delirium and their relevance for daily clinical practice: a
systematic review. Int J Geriatr Psychiatry 2005;20(7):609-15. DOI:
10.1002/gps.1343.
68. Robinson TN, Raeburn CD, Tran ZV, Brenner LA, Moss M. Motor
subtypes of postoperative delirium in older adults. Arch Surg
2011;146(3):295-300. DOI: 10.1001/archsurg.2011.14.
69. Todd A, Blackley S, Burton JK, et al. Reduced level of arousal and
increased mortality in adult acute medical admissions: a systematic review
and meta-analysis. BMC Geriatr 2017;17(1):283. DOI:
10.1186/s12877-017-0661-7.
70. Garcez FB, Aliberti MJR, Poco PCE, et al. Delirium and Adverse
Outcomes in Hospitalized Patients with COVID-19. J Am Geriatr Soc
2020;68(11):2440-2446. DOI: 10.1111/jgs.16803.
71. Krewulak KD, Stelfox HT, Ely EW, Fiest KM. Risk factors and
outcomes among delirium subtypes in adult ICUs: A systematic review. J Crit
Care 2020;56:257-264. DOI: 10.1016/j.jcrc.2020.01.017.
72. Centers for Medicare & Medicaid Services. Guiding Principles
for Making Changes to Severity Levels. Washington, DC. 2020:85(182) Fed.
Reg. 58,550-58,554.
73. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically
ventilated patients: validity and reliability of the confusion assessment
method for the intensive care unit (CAM-ICU). JAMA 2001;286(21):2703-10.
DOI: 10.1001/jama.286.21.2703.
74. Han JH, Wilson A, Vasilevskis EE, et al. Diagnosing delirium in
older emergency department patients: validity and reliability of the
delirium triage screen and the brief confusion assessment method. Ann Emerg
Med 2013;62(5):457-465. DOI: 10.1016/j.annemergmed.2013.05.003.
75. Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: development and
validation of a new scoring system for delirium severity in 2 cohorts. Ann
Intern Med 2014;160(8):526-33. DOI: 10.7326/M13-1927.
76. Vasunilashorn SM, Guess J, Ngo L, et al. Derivation and Validation
of a Severity Scoring Method for the 3-Minute Diagnostic Interview for
Confusion Assessment Method--Defined Delirium. J Am Geriatr Soc
2016;64(8):1684-9. DOI: 10.1111/jgs.14234.
77. Khan BA, Perkins AJ, Gao S, et al. The Confusion Assessment Method
for the ICU-7 Delirium Severity Scale: A Novel Delirium Severity Instrument
for Use in the ICU. Crit Care Med 2017;45(5):851-857. DOI:
10.1097/CCM.0000000000002368.
78. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz
RI. Clarifying confusion: the confusion assessment method. A new method for
detection of delirium. Ann Intern Med 1990;113(12):941-8.
(http://www.ncbi.nlm.nih.gov/pubmed/2240918).
79. Motyl CM, Ngo L, Zhou W, et al. Comparative Accuracy and
Efficiency of Four Delirium Screening Protocols. J Am Geriatr Soc
2020;68(11):2572-2578. DOI: 10.1111/jgs.16711.
80. Marcantonio ER, Fick DM, Jung Y, et al. Comparative Implementation
of a Brief App-Directed Protocol for Delirium Identification by
Hospitalists, Nurses, and Nursing Assistants : A Cohort Study. Ann Intern
Med 2022;175(1):65-73. DOI: 10.7326/M21-1687.
81. Fuchs S, Bode L, Ernst J, Marquetand J, von Kanel R, Bottger S.
Delirium in elderly patients: Prospective prevalence across hospital
services. Gen Hosp Psychiatry 2020;67:19-25. DOI:
10.1016/j.genhosppsych.2020.08.010.
82. Oldham MA. Connecting acute and chronic neurocognitive impairment.
Int Psychogeriatr 2022;34(4):323-325. DOI: 10.1017/S104161022200028X.
83. Lee HB, Oldham MA, Sieber FE, Oh ES. Impact of Delirium After Hip
Fracture Surgery on One-Year Mortality in Patients With or Without Dementia:
A Case of Effect Modification. Am J Geriatr Psychiatry 2017;25(3):308-315.
DOI: 10.1016/j.jagp.2016.10.008.
84. First M. New ICD-10-CM Codes for Neurocognitive Disorders
Effective October 1. Psychiatric News. American Psychiatric Association. 24
August 2022.
85. Flaherty JH, Rudolph J, Shay K, et al. Delirium is a serious and
under-recognized problem: why assessment of mental status should be the
sixth vital sign. J Am Med Dir Assoc 2007;8(5):273-5. DOI:
10.1016/j.jamda.2007.03.006.
86. Flaherty JH, Shay K, Weir C, et al. The development of a mental
status vital sign for use across the spectrum of care. J Am Med Dir Assoc
2009;10(6):379-80. DOI: 10.1016/j.jamda.2009.04.001.
87. Fick DM, Shrestha P. Delirium in Persons With Dementia:
Integrating the 4Ms of Age-Friendly Care as a Set Into the Care of Older
People. J Gerontol Nurs 2022;48(10):3-6. DOI: 10.3928/00989134-20220909-01.
88. Katlic MR, Robinson TN. The Costs of Postoperative Delirium. JAMA
Surg 2021;156(5):470-471. DOI: 10.1001/jamasurg.2020.7257.
89. Kosar CM, Thomas KS, Inouye SK, Mor V. Delirium During Postacute
Nursing Home Admission and Risk for Adverse Outcomes. J Am Geriatr Soc
2017;65(7):1470-1475. DOI: 10.1111/jgs.14823.
90. Evered L, Silbert B, Knopman DS, et al. Recommendations for the
Nomenclature of Cognitive Change Associated with Anaesthesia and
Surgery-2018. Anesthesiology 2018;129(5):872-879. DOI:
10.1097/ALN.0000000000002334.
91. Marcantonio ER. Postoperative delirium: a 76-year-old woman with
delirium following surgery. JAMA 2012;308(1):73-81. DOI:
10.1001/jama.2012.6857.
92. Inouye SK. Joining Forces against Delirium - From Organ-System
Care to Whole-Human Care. N Engl J Med 2020;382(6):499-501. DOI:
10.1056/NEJMp1910499.
93. Ross IR. Through the Fog. N Engl J Med 2021;384(20):1882-1883.
94. Oldham MA, Slooter AJC, Ely EW, Crone C, Maldonado JR, Rosenthal
LJ. An Interdisciplinary Reappraisal of Delirium and Proposed Subtypes. J
Acad Consult Liaison Psychiatry 2023;64(3):248-261. DOI:
10.1016/j.jaclp.2022.07.001.
95. Burry L, Hutton B, D. W, et al. Medicines to treat delirium in
critically ill adult patients. Cochrane Database Syst Rev 2019(9):CD011749.
96. O'Mahony R, Murthy L, Akunne A, Young J, Guideline Development G.
Synopsis of the National Institute for Health and Clinical Excellence
guideline for prevention of delirium. Ann Intern Med 2011;154(11):746-51.
DOI: 10.7326/0003-4819-154-11-201106070-00006.
97. Soiza RL, Myint PK. The Scottish Intercollegiate Guidelines
Network (SIGN) 157: Guidelines on Risk Reduction and Management of Delirium.
Medicina (Kaunas) 2019;55(8). DOI: 10.3390/medicina55080491.
98. NSW: Australian Commission on Safety and Quality in Health Care.
Delirium Clinical Care Standard. 2021.
99. Aldecoa C, Bettelli G, Bilotta F, et al. European Society of
Anaesthesiology evidence-based and consensus-based guideline on
postoperative delirium. Eur J Anaesthesiol 2017;34(4):192-214. DOI:
10.1097/EJA.0000000000000594.
100. Martin J, Heymann A, Basell K, et al. Evidence and consensus-based
German guidelines for the management of analgesia, sedation and delirium in
intensive care--short version. Ger Med Sci 2010;8:Doc02. DOI:
10.3205/000091.
101. Matsuda Y, Tanimukai H, Inoue S, et al. JPOS/JASCC clinical
guidelines for delirium in adult cancer patients: a summary of
recommendation statements. Jpn J Clin Oncol 2020;50(5):586-593. DOI:
10.1093/jjco/hyaa003.
102. Lipowski ZJ. Delirium: Acute Brain Failure in Man. Springfield, IL:
Charles C Thomas, 1980.
103. Maldonado JR. Acute Brain Failure: Pathophysiology, Diagnosis,
Management, and Sequelae of Delirium. Crit Care Clin 2017;33(3):461-519.
DOI: 10.1016/j.ccc.2017.03.013.
104. Davis DH, Muniz-Terrera G, Keage HA, et al. Association of Delirium
With Cognitive Decline in Late Life: A Neuropathologic Study of 3
Population-Based Cohort Studies. JAMA Psychiatry 2017;74(3):244-251. DOI:
10.1001/jamapsychiatry.2016.3423.
105. Krogseth M, Davis D, Jackson TA, et al. Delirium, neurofilament
light chain, and progressive cognitive impairment: analysis of a prospective
Norwegian population-based cohort. Lancet Healthy Longev
2023;4(8):e399-e408. DOI: 10.1016/S2666-7568(23)00098-3.
106. Casey CP, Lindroth H, Mohanty R, et al. Postoperative delirium is
associated with increased plasma neurofilament light. Brain
2020;143(1):47-54. DOI: 10.1093/brain/awz354.
107. Ballweg T, White M, Parker M, et al. Association between plasma tau
and postoperative delirium incidence and severity: a prospective
observational study. Br J Anaesth 2021;126(2):458-466. DOI:
10.1016/j.bja.2020.08.061.
108. Wang S, Greene R, Song Y, et al. Postoperative delirium and its
relationship with biomarkers for dementia: a meta-analysis. Int
Psychogeriatr 2022:1-14. DOI: 10.1017/S104161022100274X.
109. Moyer DD. Review article: terminal delirium in geriatric patients
with cancer at end of life. Am J Hosp Palliat Care 2011;28(1):44-51. DOI:
10.1177/1049909110376755.
110. Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent
nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med
2015;175(4):512-20. DOI: 10.1001/jamainternmed.2014.7779.
111. Burton JK, Craig LE, Yong SQ, et al. Non-pharmacological
interventions for preventing delirium in hospitalised non-ICU patients.
Cochrane Database Syst Rev 2021;7:CD013307. DOI:
10.1002/14651858.CD013307.pub2.
112. Fick DM. AGS CoCare((R)): HELP Reduces In-Hospital Delirium. J
Gerontol Nurs 2023;49(8):51-52. DOI: 10.3928/00989134-20230707-02.
113. Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital Elder
Life Program: Systematic Review and Meta-analysis of Effectiveness. Am J
Geriatr Psychiatry 2018;26(10):1015-1033. DOI: 10.1016/j.jagp.2018.06.007.
114. Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for Critically Ill
Patients with the ABCDEF Bundle: Results of the ICU Liberation Collaborative
in Over 15,000 Adults. Crit Care Med 2019;47(1):3-14. DOI:
10.1097/CCM.0000000000003482.
115. Collinsworth AW, Priest EL, Masica AL. Evaluating the
Cost-Effectiveness of the ABCDE Bundle: Impact of Bundle Adherence on
Inpatient and 1-Year Mortality and Costs of Care. Crit Care Med
2020;48(12):1752-1759. DOI: 10.1097/CCM.0000000000004609.
116. O'Regan NA, Fitzgerald J, Adamis D, Molloy DW, Meagher D, Timmons
S. Predictors of Delirium Development in Older Medical Inpatients: Readily
Identifiable Factors at Admission. J Alzheimers Dis 2018;64(3):775-785. DOI:
10.3233/JAD-180178.
117. John A. Hartford Foundation, Institute for Healthcare Improvement.
Age-Friendly Health Systems.
(ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/).
118. American Geriatrics Society. HELP and the 4M's Overview.
(help.agscocare.org/fulltext/null/H00110/H00110_PART001_001/366).
119. Society of Critical Care Medicine. ICU Liberation A-F Bundle.
(www.sccm.org/iculiberation/abcdef-bundles).
120. American College of Surgeons. Geriatric Surgery Verification.
(www.facs.org/quality-programs/accreditation-and-verification/geriatric-surgery-verification).
121. American Delirium Society. American Delirium Society.
(https://americandeliriumsociety.org/).
122. Aiken LH, Lasater KB, Sloane DM, et al. Physician and Nurse
Well-Being and Preferred Interventions to Address Burnout in Hospital
Practice: Factors Associated With Turnover, Outcomes, and Patient Safety.
JAMA Health Forum 2023;4(7):e231809. DOI: 10.1001/jamahealthforum.2023.1809.