Toci Si or Toci No –The Tocilizumab Dilemma
COVID IN FOCUS: PERSPECTIVES ON THE LITERATURE
This CHEST series highlights specific studies in the COVID-19 literature that may warrant discourse or reading for members of the chest medicine community. Articles are written by members of CHEST Networks. You can read additional articles in this series.
NOTE: The perspectives shared in this article are those of the author(s) and not those of CHEST.
Toci Si or Toci No – The Tocilizumab Dilemma
By: Marcos I. Restrepo, MD, MSc, PhD, FCCP
Chest Infections Network
Published: April 19, 2021
Despite the use of corticosteroids, the mortality rate in severe and critical cases of COVID-19 remains unacceptably high. This is why the Chest Infections Network has chosen to review the literature on the use of tocilizumab (TCZ) to suggest alternatives for clinicians to improve survival among critically ill patients affected by COVID-19. Over the past months, several manuscripts have been published:
 Click image to view larger
Click image to view larger
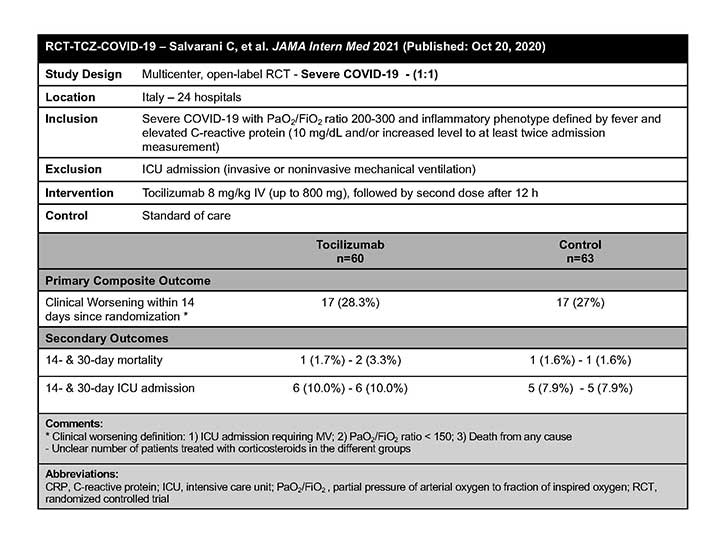
RCT-TCZ-COVID-19
In this randomized clinical trial of hospitalized adult patients with COVID-19 pneumonia and a PaO2/FiO2 ratio between 200-300 mm Hg, the RCT-TCZ-COVID-19 authors concluded that no benefit on disease progression was observed in patients who received TCZ compared with standard care.1
 Click image to view larger
Click image to view larger
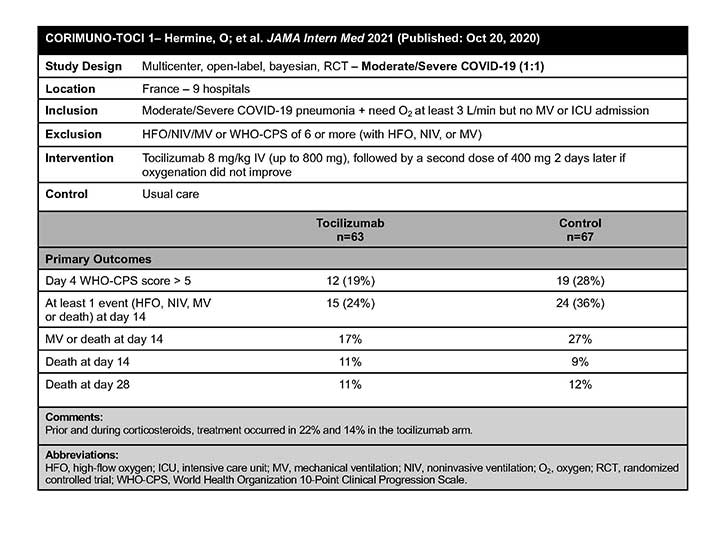
CORIMUNO-TOCI 1
The CORIMUNO-TOCI 1 authors concluded that TCZ did not reduce WHO-CPS scores lower than 5 at day 4 in patients with COVID-19 pneumonia requiring supplemental oxygen support but who were not admitted to the ICU.2 However, TCZ might have reduced the risk of progression to noninvasive ventilation, mechanical ventilation, or death by day 14, although no differences were found between groups on day 28.2
 Click image to view larger
Click image to view larger
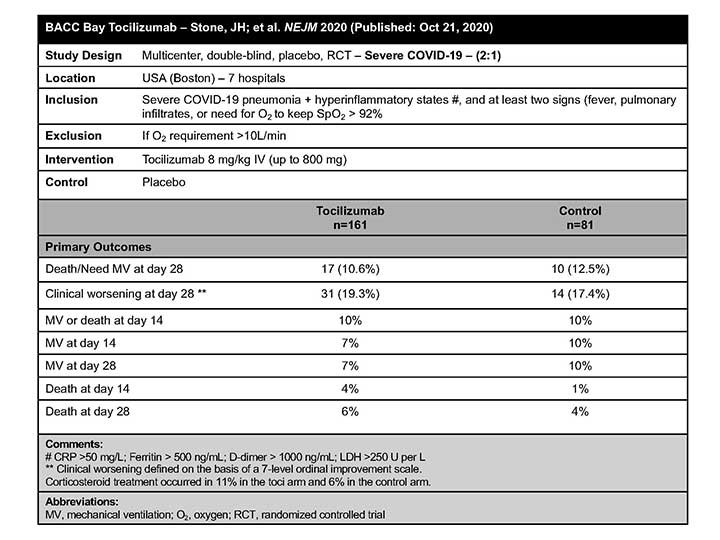
BACC Bay
In a study population of adult inpatients with moderate to severe COVID-19 not requiring intensive care, of whom 80% required supplemental oxygen at enrollment, the BACC Bay Tocilizumab Trial Investigators concluded that TCZ was not effective for preventing intubation or death.3
 Click image to view larger
Click image to view larger
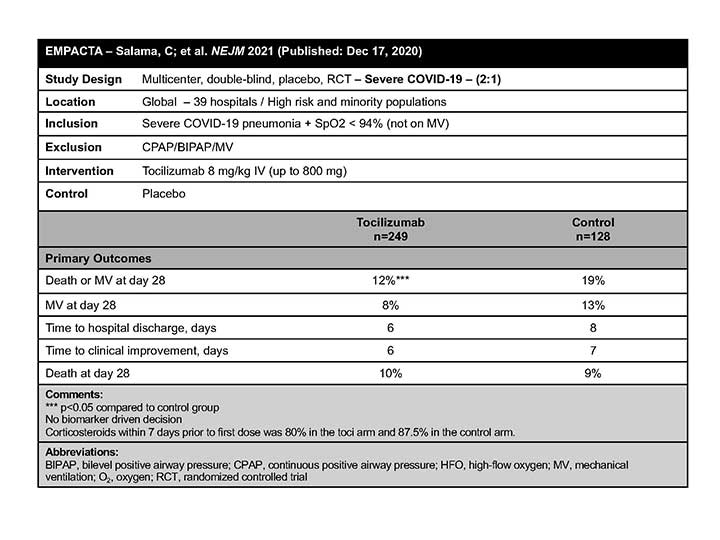
EMPACTA
The EMPACTA authors concluded that TCZ reduced the likelihood of progression to the composite outcome of mechanical ventilation or death, but it did not improve survival in hospitalized patients with COVID-19 pneumonia who were not receiving mechanical ventilation at enrollment.4
 Click image to view larger
Click image to view larger
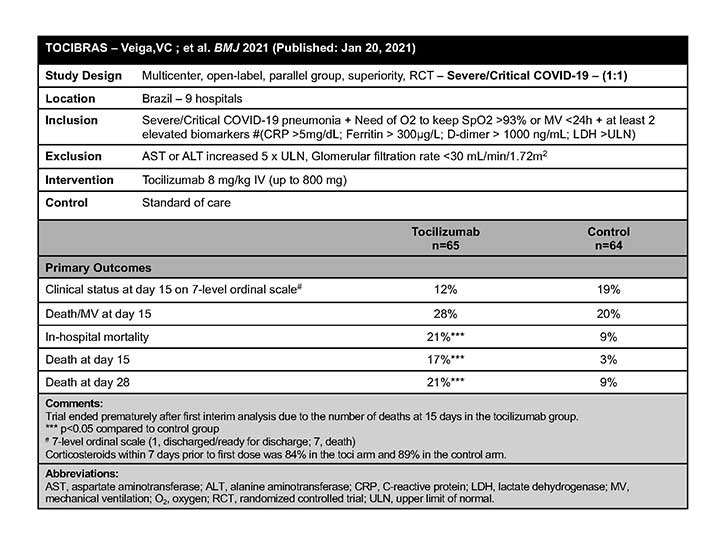
TOCIBRAS
The TOCIBRAS authors concluded that in patients with severe or critical COVID-19, TCZ plus standard of care was not superior to standard of care alone in improving clinical outcomes at 15 days, and it might increase mortality.5
 Click image to view larger
Click image to view larger
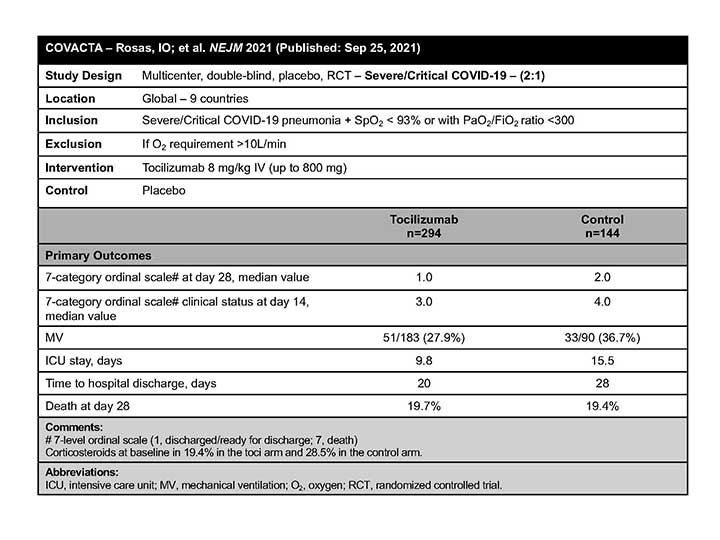
COVACTA
The COVACTA investigators concluded that TCZ did not improve clinical status or mortality in severe/critically ill patients with COVID-19 pneumonia.6
 Click image to view larger
Click image to view larger
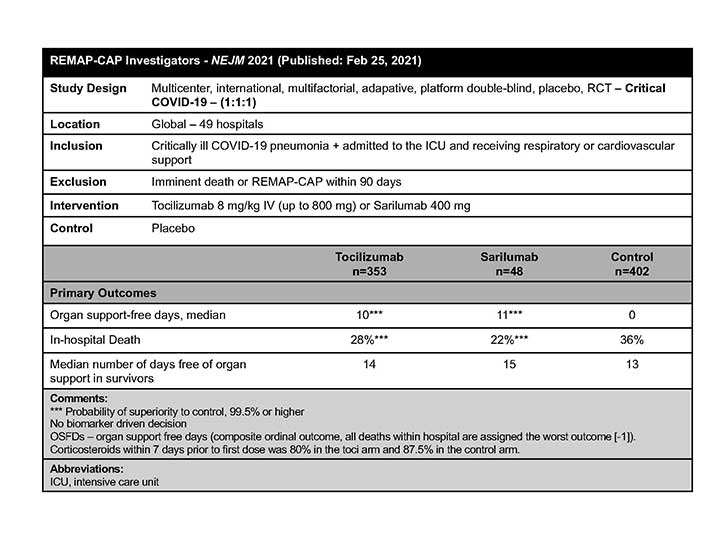
REMAP-CAP
The REMAP-CAP Investigators concluded that in critically ill patients with COVID-19 receiving organ support in the ICU, treatment with one of two IL-6 receptor antagonists—TCZ and sarilumab—improved outcomes, including survival.7
 Click image to view larger
Click image to view larger
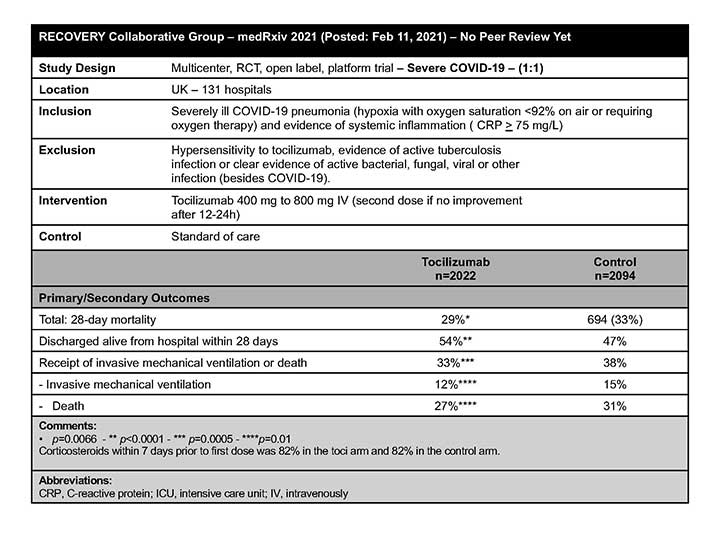
RECOVERY
In a preprint article published before peer review, the RECOVERY Collaborative Group concluded that in hospitalized patients with COVID-19 who experienced hypoxia and systemic inflammation, the administration of TCZ additional to systemic corticosteroids improved survival and other clinical outcomes (eg, were more likely to be discharged from the hospital alive within 28 days).8
What are the key takeaways from these trials?
- Current standard of care for patients requiring oxygen therapy includes the use of corticosteroids. Therefore, the potential beneficial effect seems to be closely related to the combination of corticosteroids and TCZ.
- The differences in mortality among the varied TCZ studies suggest that there is major heterogeneity that makes strong conclusions complex. However, the data seem to favor a benefit with TCZ in those patients hospitalized with COVID-19 who require noninvasive (including high-flow oxygen therapy) or invasive mechanical ventilation.
- However, there is a consideration to use TCZ in patients who are highly inflamed (based on C-reactive protein values) and rapidly progressing to high oxygen requirements.
- Tocilizumab has been well tolerated, without a higher risk of infections compared with what was initially thought based on observational studies.
- Patients with immunosuppression were excluded or were underrepresented in the above-mentioned studies, limiting the understanding of possible benefit or harm with the use of TCZ in these patients.
References
- Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):24-31.
- Hermine O, Mariette X, Tharaux P, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):32-40.
- Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with COVID-19. N Engl J Med. 2020;383:2333-2344.
- Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N Engl J Med. 2021;384;20-30.
- Veiga VC, Prats JAGG, Farias DLC, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2021;372:N84.
- Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe COVID-19 pneumonia. N Engl J Med. 2021.
- The REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med; 2021.
- Horby PW, Pessoa-Amorim G, Peto L, et al; RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. medRxiv. 2021.
Marcos I. Restrepo, MD, MSc, PhD, FCCP
• Professor at the Department of Medicine, Division of Pulmonary/Critical Care Medicine, and Associate Program Director of the Pulmonary/Critical Care Medicine fellowship program at the University of Texas Health San Antonio
• Investigator and Medical Director of the Medical Intensive Care Unit at the South Texas Veterans Health Care System, Audie L. Murphy Division
• Member of the journal CHEST® Editorial Board